Your Partner in Emergency Safety
We develop powered anti-choking devices to help families and institutions respond quickly.

Our core goal
 Simplicity
Simplicity
We design for ease-of-use so anyone can act quickly in emergencies.
 Reliability
Reliability
We ensure dependable performance when every second counts.
 Independent
Independent
We create tools that empower self-rescue without outside help.
 Safety
Safety
We integrate multiple safety layers to protect every user.
 Innovation
Innovation
We continually improve anti-choking technology for better outcomes.

About Us
TIGAS
-
Tigas is dedicated to developing an intelligent powered anti-choking device that helps families and institutions respond quickly to sudden airway blockages through self-rescue or assisted rescue.
Based in Hong Kong, serving households, schools, and institutions worldwide.
Certificate
FDA Registration
Registered as FDA Class I device, compliant with 21 CFR 807.39.
EU MDR Registrationn
Registered under MDR (EU) 2017/745, Class I device.
Third-Party Tested
Validated by independent labs to meet safety standards.
OEM Partnerships
Offering OEM solutions outside mainland China.
Tigas Intelligent Anti-Choking Device
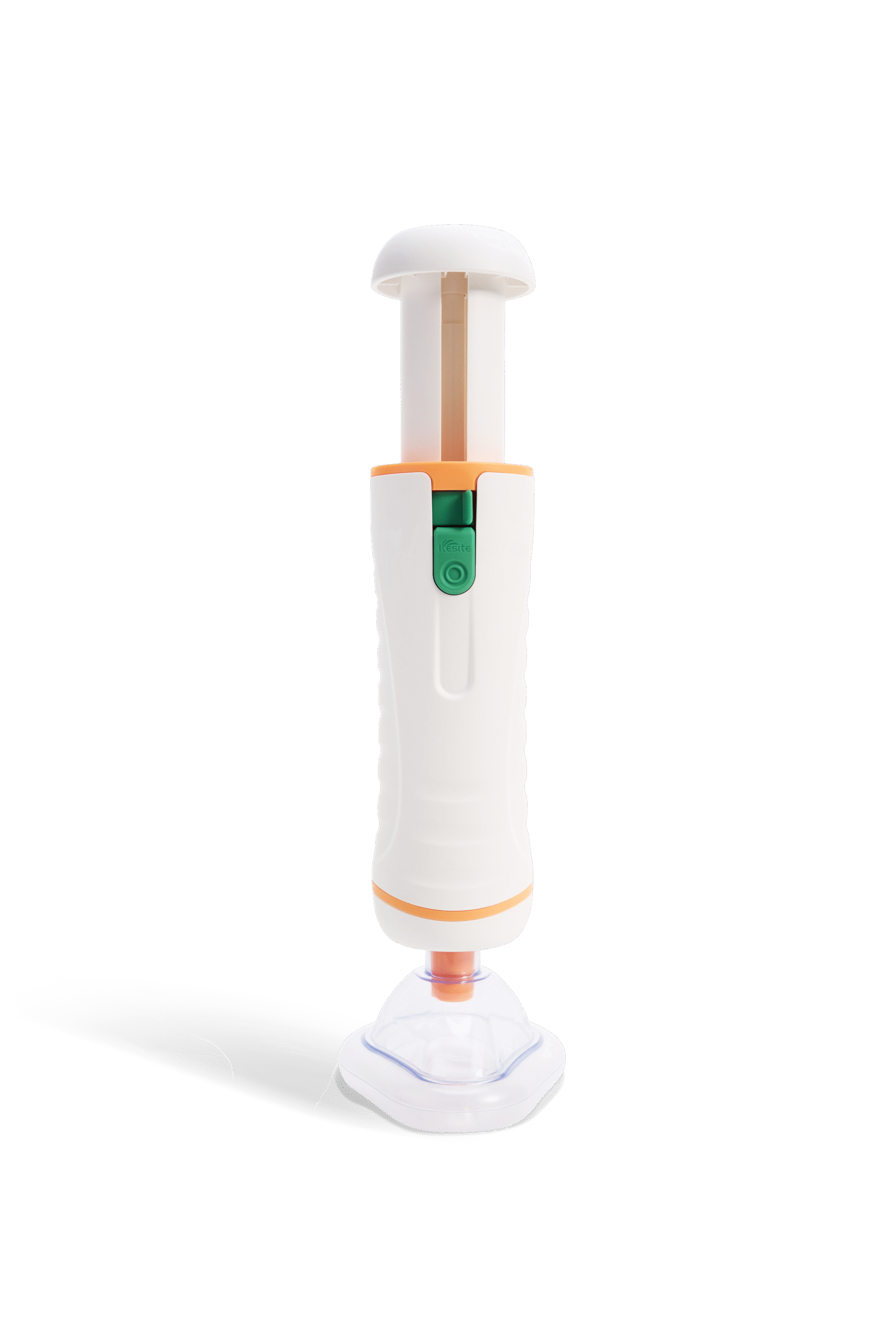
Tigas Model IA
Compact & Easy-to-Use
Compact, easy-to-use device for quick self-rescue or assisted rescue in choking emergencies.

Tigas Model IA
Designed for All Scenarios
Suitable for home, travel, schools, elder care centers, and restaurants.

Tigas Model IA
Safe for All Ages
Features one-touch suction system, anti-backflow valve, and multiple safety locks for safe use by children, adults, and the elderly.

Tigas Model IA
One-Touch Operation
One-touch operation, no professional training required.

Tigas Model IA
Stable Negative Pressure
Provides stable negative pressure for effective obstruction removal.

Tigas Model IA
Low Operating Threshold
Requires minimal effort to operate, even for weaker users.

Tigas Model IA
Reduces Risk of Secondary Injury
Safety locks and anti-reflux features help prevent accidental harm.

Tigas Model IA
Multiple Face Shield Sizes
Comes with multiple mask sizes to fit different users.

Tigas Model IA
Technical Specs – Dimensions
Design length: 279.59 mm (Actual: 283 mm).
Design width: 78.2 mm (Actual: 78 mm).

Tigas Model IA
Technical Specs – Performance
Pressure range: 30 kPa – 39 kPa Spring parameters: 3×180×50 mm

Tigas Model IA
Certifications
FDA Registered – Device Class I, Code: BSY.
EU MDR Registered – Class I, valid until 2030.
Tested by independent labs for safety compliance.

Don’t Let Choking Take a Life!
Get Tigas and be ready to save a life today!
Frequently Asked Questions
Solution
What is the Tigas device used for?
Assist families and institutions in achieving rapid self rescue or rescue in the event of sudden suffocation, reducing the risk of preventable death caused by airway obstruction.
How do I buy the Tigas device?
The product is currently sold globally through Shopify's official website and provides stable delivery support and customized solutions for households and small and medium-sized institutions such as nursing homes, schools, and food chains.
Is the Tigas device certified?
Tigas' cooperative production enterprise Chongqing Kesite Medical Technology Co., Ltd. has completed the registration of Device Class I, Code: BSY with the US FDA, registration number D564289, valid until December 31, 2025. In addition, to meet the requirements of the EU MDR (2017/745) regulation, Tigas products have also completed the European CE registration process. The authorized representative in Europe is Debao International GmbH, registration number DE/CA30/00208798, product classification is Class I, and the certification is valid until May 3, 2030 CE receipt.
Does Tigas offer OEM service?
In addition to Tigas private brand sales, we also provide OEM cooperation mode for markets outside Chinese Mainland. Whether you are a medical equipment brand, an emergency product channel provider, or an organization looking to customize localized solutions, we welcome in-depth negotiations to jointly promote safe and effective intelligent anti suffocation products.
How can I apply for brand cooperation?
If you wish to learn more about product details, apply for cooperative agency, obtain customized quotations or technical support, please feel free to contact the Tigas team through the Contact Us page. We support email and phone consultations, and customer service personnel will respond promptly within working days in Hong Kong time zone to ensure that every issue is taken seriously and effectively resolved.